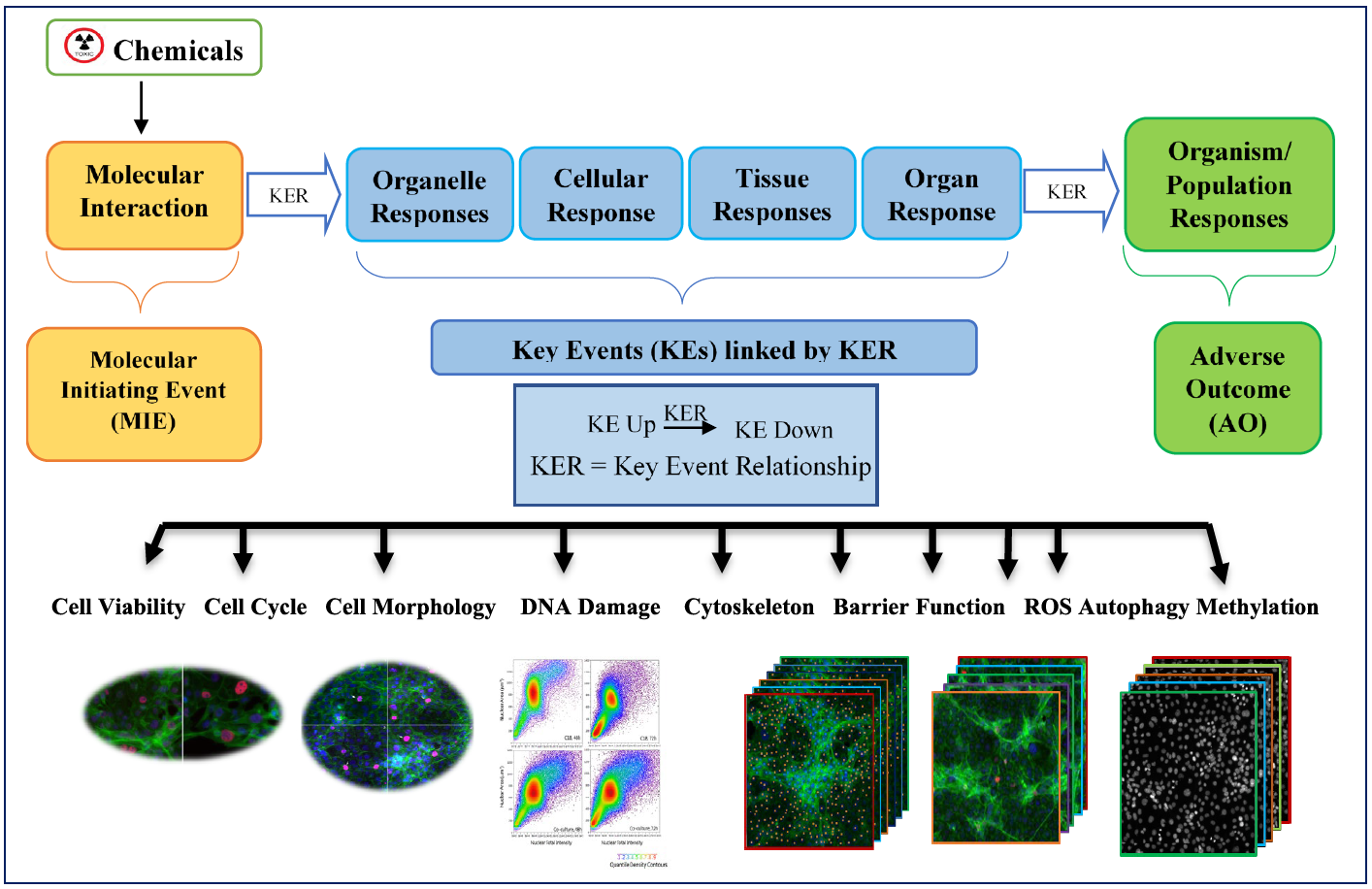
Our Technology
Reprotox through our expertise has developed a proprietary
technology to establish in vitro 3D testicular model for mice,
rats and canine. We produce the 3D model from neonate, juvenile
and adolescent, representing differential stages of
spermatogenesis. This cost-effective and time-efficient system
allows the study of male reproductive health with respect to
various drug and toxicants. Our team continually works to
improve these models, and works with clients to achive their
goals.
3D Testis Model System
Reprotox through our expertise has developed
an animal-free murine in vitro 3D culture model using testicular
cell lines including Legdig, Sertoli and germ cells. We have
confirmed the effectiveness of this model (Yin et al. 2017).
This system allows the study of male reproductive health with
respect to various toxicants.
Reprotox has developed a proprietary
technology to establish in vitro 3D testicular model at
different stage of spermatogenesis from mice, rats and canine.
We derived testicular cells from neonate, juvenile and
adolescent, established these testicular-like organiods
representing differential stages of spermatogenesis. This
organoid culture recapitulate various features of
spermatogenesis in vivo, and enable assessment of
differentiation and mitosis. This technology provides a
reliable, robust, and relevant in vitro system for preclinical
drug discovery, repro-toxicity assessment, and disease modeling.